DNA Diagnostics Center (DDC) is one of the leading and most highly-accredited genetic-testing laboratories in the world. We provide comprehensive DNA testing services for:
About DNA Diagnostics Center (DDC)
Helping people get answers through expert DNA testing and other personal diagnostics.

DNA Answers that Drive You Forward
- DNA paternity testing
- Immigration DNA testing
- DNA relationship testing
- Fertility testing
- Animal breeders and pet testing
- DNA lifestyle testing
As scientists, innovators, problem-solvers, and doers, our focus is on what matters most: helping our customers get the answers they need to life’s most important questions.
Who We Are
In 1995, DDC was founded on the belief that the technological advancements in DNA testing should translate into services that are accessible and affordable to everyone. Today, our DNA tests are trusted by:
- Hospitals and clinics
- Government agencies
- The legal community
- Law enforcement
- Consumers in over 160 countries worldwide
Who We Are
In 1995, DDC was founded on the belief that the technological advancements in DNA testing should translate into services that are accessible and affordable to everyone. Today, our DNA tests are trusted by:
- Hospitals and clinics
- Government agencies
- The legal community
- Law enforcement
- Consumers in over 160 countries worldwide

The DDC Difference
Our People · Our Processes · Our Passion

Our People
Over 200 dedicated staff at our Cincinnati, Ohio, laboratory and at our 3,700 satellite offices across, North America, South America, Europe, Asia, and Africa come to work with one goal in mind: to help answer our customers’ important life questions by providing top-quality and accurate testing services with caring and compassionate service. It’s a challenge we proudly accept every day.

Our Processes
Since 1995, DDC scientists and service teams have established tried-and-true processes and maintained best practices in our laboratory and the company as a whole, attaining the highest levels of accreditation and a reputation in the industry that are unsurpassed. We stay up to date on the latest in genetic technology, constantly striving to improve efficiency, accuracy, and quality of our tests.

Our Passion
We endeavor to help people answer difficult questions about family relationships or their health, and it takes energy and resolve from everyone at DDC to develop helpful products and provide accurate results that are often life-changing. Many employees have been with the company for decades because they are committed to making a difference for fellow humans in need.
Our Leadership Team

Jason has a deep background and passion for excellence in delivering quality products to the marketplace in a cost-effective and predictable way. Jason has successfully led the company through transformations of performance via systems and culture and employs efficient, compliant, business solutions to ensure the successful development of management teams and broad ranging infrastructure systems.
Prior to joining DDC in 2007, Jason owned and operated a private counseling practice and managed the Crisis Counseling unit at the Mental Health & Recovery Services of Warren County, Ohio.

Kathy has over 30 years of laboratory management and operations experience. Prior to joining DDC, Kathy held various positions of increasing responsibility, with the most recent being Director of Operations for Orchid Cellmark at the Dayton facility. Kathy holds a BS degree in Medical Technology from the University of Dayton and is certified as a Medical Technologist by the American Society for Clinical Pathology (ASCP).

Prior to that, she was Vice President of Marketing for Aspire Bariatrics, a venture-backed medical device company, where she played an integral role in successfully bringing a novel implantable device from concept through FDA approval and global commercialization. Previous roles include sales and business development at Synthes, a global orthopedics company acquired by Johnson & Johnson for $21B, and product marketing at Medtronic. Christy received her MBA from The Wharton School (U of Penn), and her BA in Economics from Princeton University.

Debra has more than 25 years of experience in Paternity and Relationship testing. She holds a PhD in Biomedical Sciences with concentration in Molecular Genetics from Wright State University and a Post-Doc in Molecular Biology research at the University of Cincinnati. She has served as a member of the AABB RT Accreditation Subcommittee 2018 and is a past member of the AABB RT Standards Subcommittee. Prior to joining DDC in 2012, Debra was a Laboratory Director at Orchid Cellmark in Dayton, Ohio for 14 years.
Our DDC Laboratory
- Nearly 1 million samples analyzed and processed each year
- Trusted by American Pregnancy Association, College of American Pathologists, CLIA, AABB (US), SCC (Canada), and other government agencies, legal professionals, and healthcare practitioners.
- Staffed by the leading scientists, researchers, PhDs, and experts in biology, and genetics who provide superior DNA analysis and results.

DDC Dual Process™
DDC was the first lab to require every postnatal paternity DNA test to be performed twice—by independent teams—as an extra layer of accuracy assurance. At each step during testing, samples are tracked, identified, checked against our records, and then a staff member signs off at each point. The Association for the Advancement of Blood & Biotherapies (AABB) honored DDC with its Commendable Practice Award for implementing this process.
Advanced DNA Testing Services
Offering precision genetics analysis & personalized insights
Microsatellite Analysis
DNA Diagnostics Center (DDC) performs over 1 million STR genotyping tests annually using capillary electrophoresis platforms: ABI 3730 and 3130.

Automated Precision
Accurately processing thousands of DNA samples daily requires the use of automated precision instrumentation. The DDC laboratory uses Biomek® FXp and Biomek® 4000 systems to purify and prepare the samples before the actual DNA test.

Subsequent Analysis
DDC has invested in technology that uses the polymerase chain reaction (PCR) process that revolutionized DNA testing. We have further refined and customized our methods to provide seamless and faster sample processing, using the ProFlex™ 96-well PCR System.

Capillary Electrophoresis
Our laboratory is equipped with ABI Prism® genetic analyzers to process the large volume of cases we receive every day. These instruments perform one of the most essential steps in DNA testing: generating data through capillary electrophoresis.
Our DNA Testing Accreditations
Certified, Reliable, and Trusted Expertise
Built on a foundation of excellence
Our laboratory and scientific team have earned the highest accreditations available for genetic testing, and DDC participates in several voluntary accreditation programs.
These highly regarded compliance programs verify:
- The validity of our testing methods
- The reliability of our equipment
- The qualifications and competency of our laboratory staff, scientists, and technicians
- The soundness of our testing methods and standard operating procedures
Accreditations apply to legal DNA tests under scope.

Association for the Advancement of Blood & Biotherapies (AABB)
(formerly American Association of Blood Banks)
The AABB’s Relationship Testing Accreditation program is the international gold standard for DNA paternity testing laboratories. The program establishes and promotes the highest standards of testing quality and care for patients and clients in all aspects of parentage and other relationship testing. Most courts and other government agencies mandate that DNA tests be performed by AABB-accredited laboratories. DDC received its initial AABB accreditation in 1996 and received AABB’s commendable practice recognition in 2004 for our Dual Process™.

ANAB ISO/IEC 17025
(formerly ACLASS)
ANAB is an international laboratory accreditation organization and is signatory of the IAF and IAAC multilateral cooperative arrangements (MLAs). Through the IAF, ILAC, IAAC, and APLAC MRAs/MLAs and the Multilateral Cooperative Accreditation Arrangement, ANAB cooperates with other accreditation bodies around the world ensuring that accredited certificates are recognized nationally and internationally. ISO/IEC 17025 is the international standard set for ensuring the technical competency of laboratories and covers every aspect of laboratory management including to name a few, sample preparation, analytical testing proficiency, record keeping and reports.

College of American Pathologists (CAP)
The CAP Laboratory Accreditation Program is recognized by the federal government as being equal to or more stringent that the government’s own laboratory inspection program. DDC has maintained its accreditation under the CAP program since 2004. In addition to on-site laboratory inspection, CAP accreditation requires laboratories to participate in its Proficiency Testing Program in which CAP sends samples to the lab three times a year for DNA testing and evaluates the lab’s results. DDC has participated in the proficiency program since 2004 and has had 100% accuracy with the expected relationship interpretation on all surveys.

ISO
DDC is assessed by three different and independent accrediting organizations, using technical experts in the field, to the ISO/IEC 17025 international standards for the technical competence of calibration and testing labs.

National Association of Testing Authorities, Australia (NATA)
NATA is a national organization that provides the independent assurance of the technical competence of a laboratory meeting stringent international and Australian standards. The NATA accreditation provides a foundation of confidence in DDC’s ability to provide consistently reliable and accurate testing. NATA lists DDC/DNA Diagnostics Center as an approved laboratory, and accepts results from laboratories accredited by NATA under scope. DDC adheres to both the Australian Family Law Act Regulations 1984 and the international ISO/IEC 17025 standards.

Ministry of Justice
The MOJ is a ministerial department that works to protect the public and reduce reoffending while providing a more effective, transparent, and responsive criminal justice system for victims and the public in the United Kingdom. DNA Diagnostics Center has been accredited by the Ministry of Justice as a body that may carry out parentage tests directed by the civil courts in England and Wales under Section 20 of the Family Law Reform Act 1969.

New York State Department of Health (NYSDOH)
The NYSDOH certification permits laboratories to perform DNA tests on cases originating from New York. The New York State Department of Health rigorously inspects the entire testing service, including processes performed outside the lab such as chain of custody and patient referrals.

Standards Council of Canada (SCC)
The SCC is Canada’s national accreditation body. Because SCC accreditation is based on internationally recognized criteria (ISO/IEC 17025), SCC accreditation provides confidence and credibility in both Canada and abroad. The Citizenship and Immigration Canada (CIC) lists DDC/DNA Diagnostics Center as an approved laboratory, and accepts results from laboratories accredited by SCC under scope. Labs are not required to have a physical presence in Canada to be accredited by SCC, and select laboratories like DDC may carry out DNA analysis and retain personal information outside of Canada.

Clinical Laboratory Improvement Amendments (CLIA)
The CLIA accreditation issued by the US Department of Health ensures laboratory results are timely, accurate, and reliable. DDC has been accredited by CLIA for over 19 years.
Our Sister Companies
Peekaboo Gender Test
The only six-week blood gender test endorsed by the American Pregnancy Association and over 99% accurate.
SpermCheck
Take control of your fertility with SpermCheck at-home sperm count tests. Trusted by over 1 million couples.
DNA Diagnostics Centre
Get expert DNA answers from our UK labs. Testing is quick, private, and reliable.
empowerDX
Easy at-home health testing. Get accurate wellness, environmental, and genetic testing.
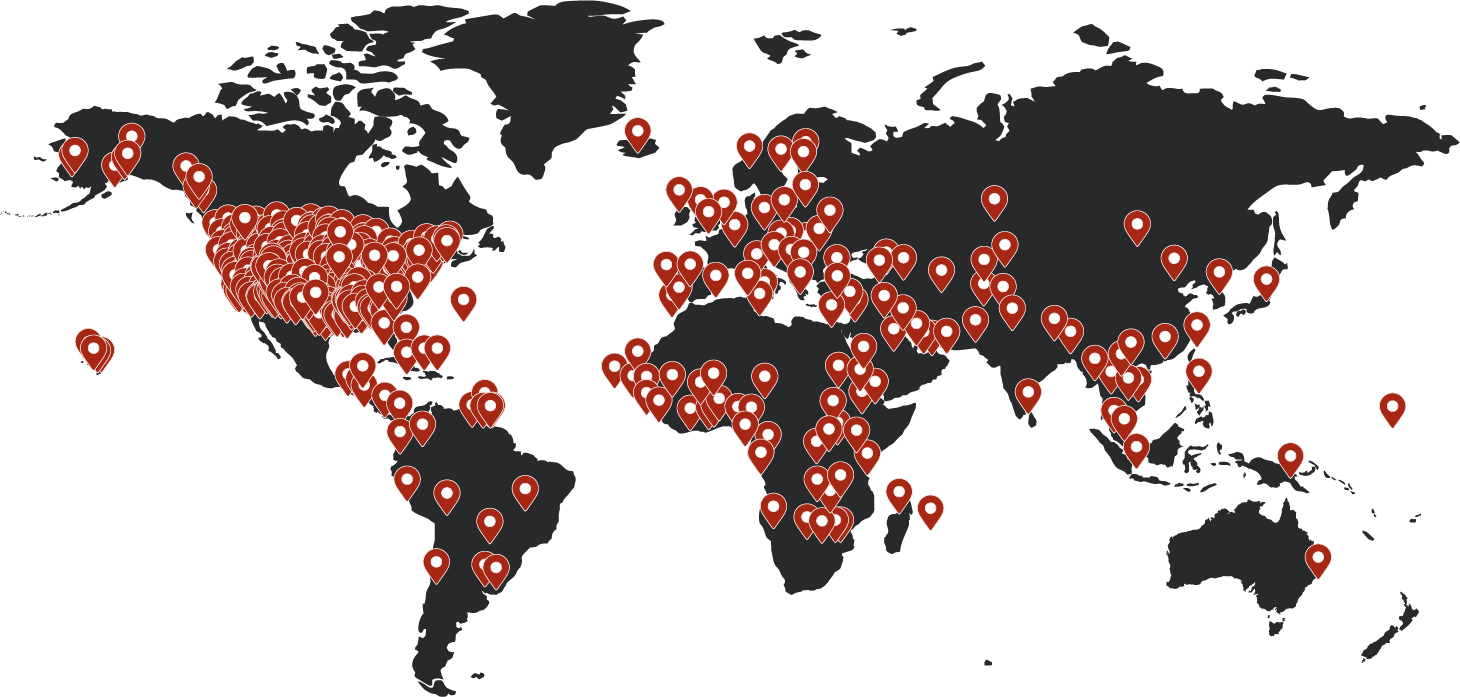
World leaders in DNA testing
Explore our global network of 5,000+ DNA collection locations across the U.S. and beyond.






